Crohn’s disease is a chronic inflammatory condition of the gastrointestinal tract, presenting a significant challenge for patients and healthcare providers. The complexity of the disease, with its unpredictable flare-ups and remissions, necessitates ongoing research to develop more effective treatments.
Crohn’s Disease Clinical Trials play a crucial role in this process, as they are the primary means of testing and optimizing new therapies. These trials are methodically structured to assess new interventions’ safety, efficacy, and potential impact, which may range from novel pharmaceuticals to dietary modifications or surgical procedures.
Participating in a clinical trial for Crohn’s disease can be a meaningful opportunity for individuals with Crohn’s disease to access cutting-edge treatments before they are widely available.
Prospective participants need to be well-informed about the potential benefits and risks, the commitment involved, and the detailed criteria for enrollment.
What is Crohn’s Disease
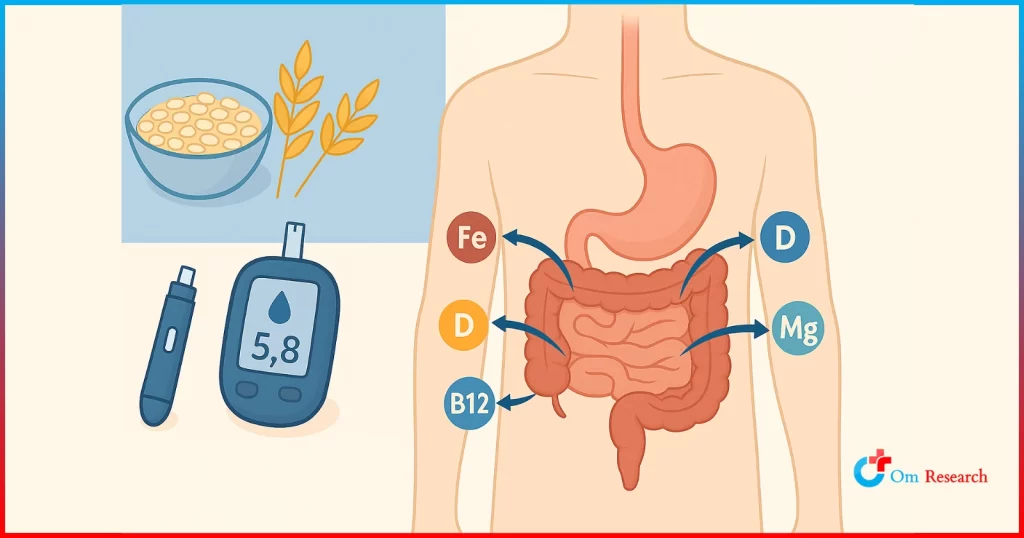
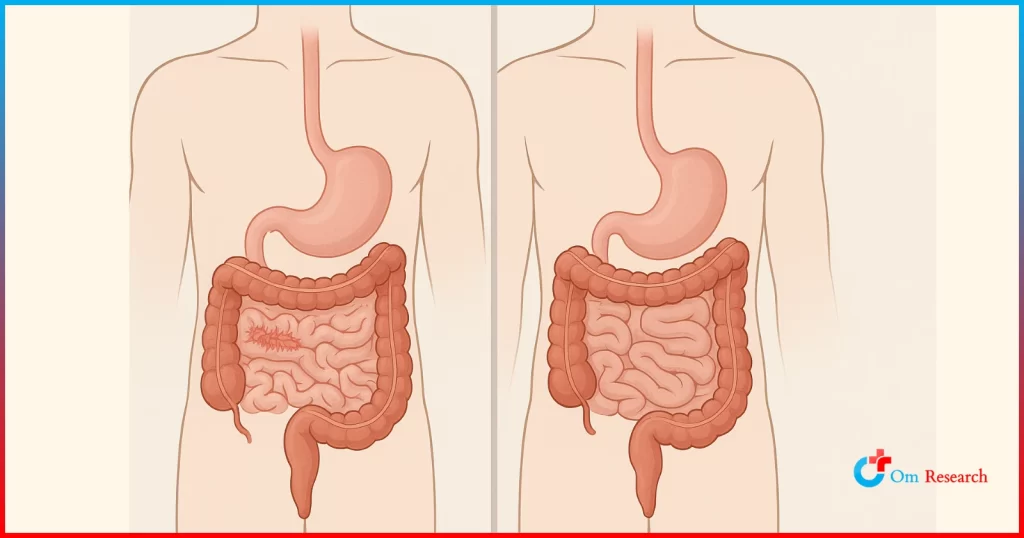
Crohn’s disease is a lifelong condition that causes digestive system inflammation (mouth, stomach, and intestines).
Symptoms
Crohn’s disease is a type of inflammatory bowel disease (IBD) that can affect any part of the gastrointestinal tract, from the mouth to the anus. Typical symptoms include persistent diarrhea, abdominal cramps, blood in the stool, weight loss, and tiredness.
Causes and Risk Factors of Crohn’s Disease
While the exact cause of Crohn’s disease remains unknown, research indicates a combination of genetic predisposition, immune system malfunctions, and environmental factors.
Risk factors include a family history of IBD, smoking, and certain medications.
Diagnostics of Crohn’s Disease
Many other available treatments for Crohn’s disease (CD) involve infusions (into a vein) or injections (under the skin), which can be uncomfortable for some people. These treatments help some people but not others, and sometimes the symptoms may come back.
Past research in patients with ulcerative colitis suggests that this investigational drug may reduce digestive system inflammation. It is hoped that it will improve CD symptoms.
Diagnosing Crohn’s disease involves a variety of tests, such as lab tests, Intestinal endoscopy, Colonoscopy, Upper GI endoscopy and enteroscopy, capsule endoscopy, Upper GI series, and CT scan. These diagnostics are crucial for tailoring treatment strategies in clinical trials.
Clinical Trials for Crohn’s Disease
Clinical trials are fundamental in advancing treatment for Crohn’s disease, providing insights into the efficacy and safety of potential therapies. By joining a clinical research study at Om Research, you can help advance research for Chron’s Disease.
Purpose and Types
Clinical trials for Crohn’s disease serve the primary purpose of testing new treatments and drugs to assess their effectiveness and safety for patients. They fall into several types:
Interventional Trials
Where new treatments are compared to existing ones or a placebo.
Observational Trials
Where outcomes in patients receiving standard treatments are monitored.
Preventive Trials
Which investigate ways to prevent disease in people who have not yet developed symptoms.
Participation in Clinical Trials
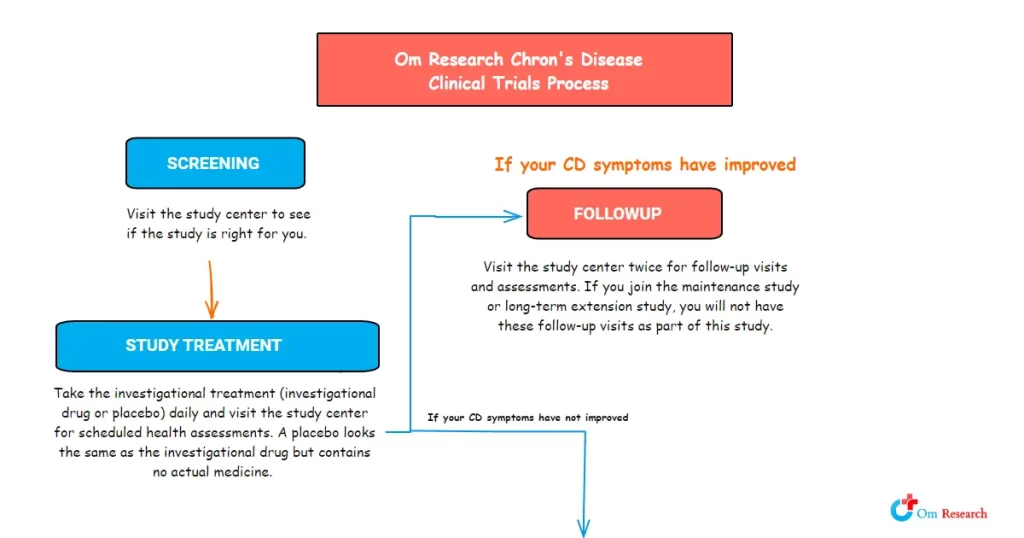
Informed Consent Process
The informed consent process is fundamental to participating in any clinical trial. This process involves detailed discussions that ensure participants understand the scope of the study, including its purpose, duration, required procedures, and key contacts.
Eligibility for Participation
You may be able to take part in the study if you*
- Are 18 to 80 years of age
- Were diagnosed with moderately to severely active CD at least 3 months ago
- Did not respond to, are no longer responding to, or did not tolerate another CD therapy
Role of Placebos
Placebos play a crucial role in clinical trials for Crohn’s disease, especially in double-blind, randomized controlled trials.
They are used as a control to help determine if the drug’s effect is truly due to the treatment and not to the patient’s belief in or expectations of the treatment. Comparing a new treatment to a placebo can highlight the new treatment’s specific impacts and side effects on Crohn’s disease progression or remission.
What else You Need to Know?
You will get complete information: The study team from Om Research will explain the possible benefits and risks of the study.
Care and Medication Provided: All the investigational treatment and study-related care will be provided to you at no cost.
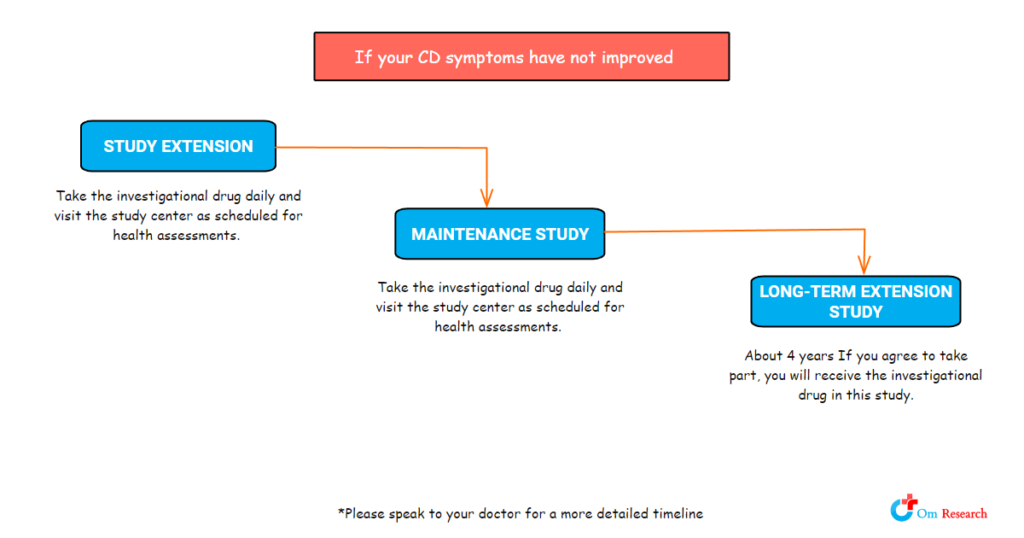
Investigational Treatment: Investigational Drug Versus Placebo: During the study treatment period, you will have a 2-in-3 chance of receiving the investigational drug and a 1-in-3 chance of receiving the placebo. During the long-term extension study, all participants will receive the investigational drug.
Reasons to participate
- Potentially get access to the oral, once-daily, investigational drug at no cost
- Be monitored by gastrointestinal (GI) specialists
- Feel empowered
- Help advance CD research
Clinical studies give medical professionals information about conditions, patients, and treatments.
Without clinical studies, there would not be any new medications. By joining this study, you will help further research and may help bring a new medication option to people with CD.
What is involved?
If you participate, your study participation could be between 7 months and just over five years. You would visit the study center regularly for study assessments.
Frequently Asked Questions
What are the eligibility criteria for participating in a Crohn’s disease clinical trial?
- Are 18 to 80 years of age
- Were diagnosed with moderately to severely active CD at least 3 months ago
- Did not respond to, are no longer responding to, or did not tolerate another CD therapy
How does participating in a clinical trial benefit a person with Crohn’s disease?
Participating in a clinical trial can provide access to new treatments before they are widely available and contribute to valuable research that may improve the standard of care for all individuals with Crohn’s disease.